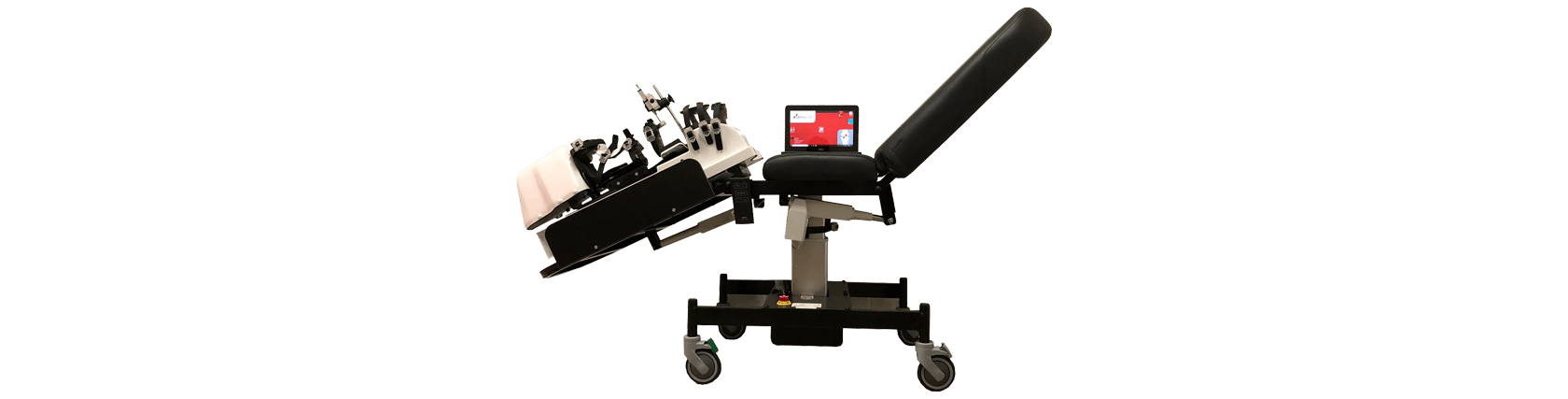
Dyneelax - Knee Arthrometer
Knee Ligament Structures Analysed: ACL, AM-PL & AL-PM Corners
Tibials Movements: Anterior Translation & Medial / Lateral Rotation
The Dyneelax knee arthrometer is an essential medical device for detailed joint stability and ligament evaluations, ideal for various medical specialists. With its ergonomic build and sophisticated measuring system, it offers dependable results and an intuitive evaluation process, raising the bar for precision in knee diagnostics & rehabilitation.
This device is an innovative medical instrument tailored for the detection, prevention, rehabilitation, and continuous observation of patients suffering from knee ligament impairments. It delivers meticulous evaluations of knee laxity by executing automated anterior drawer evaluations and rotational instability examinations, with a primary emphasis on the anterior cruciate ligament (ACL).
What is Dyneelax?
Unlock unparalleled clarity in knee ligament assessment with Dyneelax: where precision meets simplicity, redefining the gold standard in ligament diagnostics & Treatment.

Analyse the state of resistance of the ACL by applying automated anterior tibial translation (Automated Lachman Test).
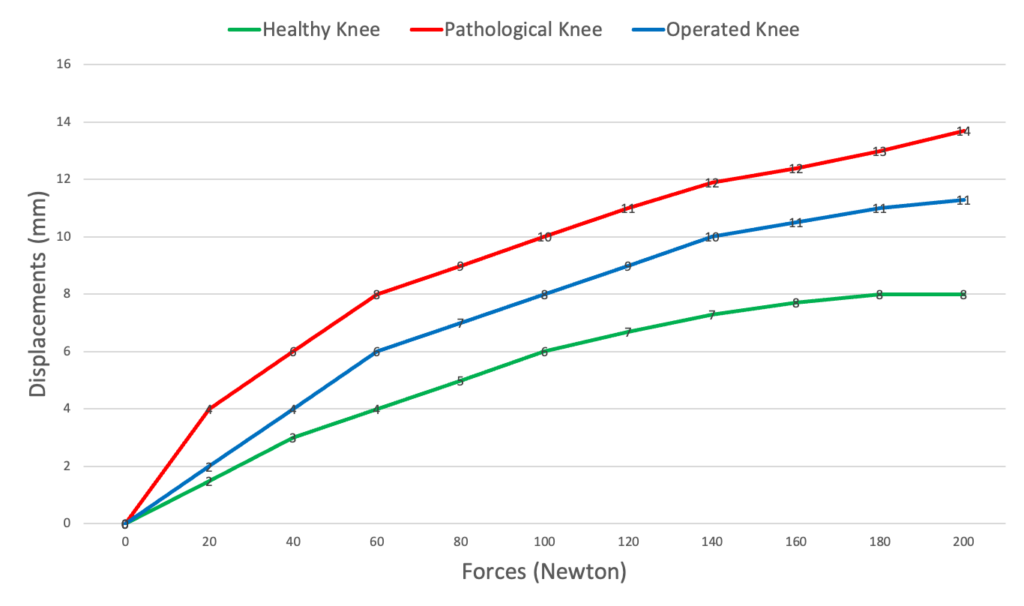
Results are shown under the form of compliance curves (force/displacement curves) + Tables Chart with displacement data.
Results are shown under the form of curves (degree/torque curves) + Tables Chart with rotation data.
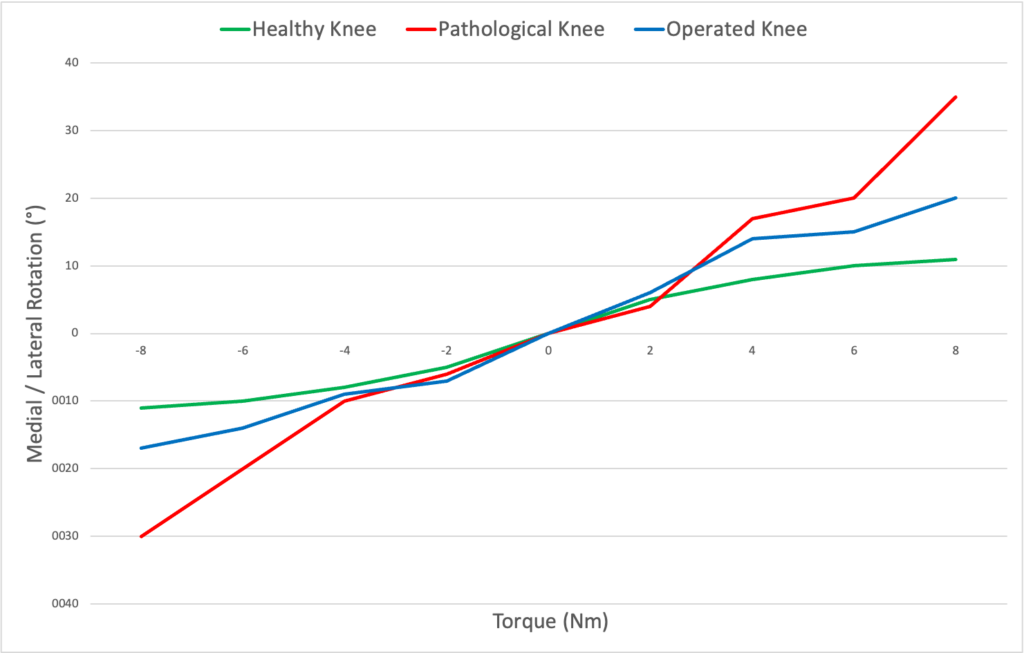
Translation findings reveal the condition of the ACL, while rotation outcomes determine the need for Extra-Articular Plasty, ensuring a well-rounded approach to treatment and recovery.

The combination of advanced sensors, defined testing parameters, and a uniform procedure delivers reproducible results that healthcare professionals can trust. This reliability enhances the precision of treatment plans, offering patients a clear path to recovery and bolstering confidence in their care.

Thanks to our system's high reproducibility, we maximize the effectiveness of early detection and preventive strategies, paving the way for ongoing follow-ups. This structured approach seamlessly transitions into rehabilitation, focusing on recovery and building strength.
Table of Contents

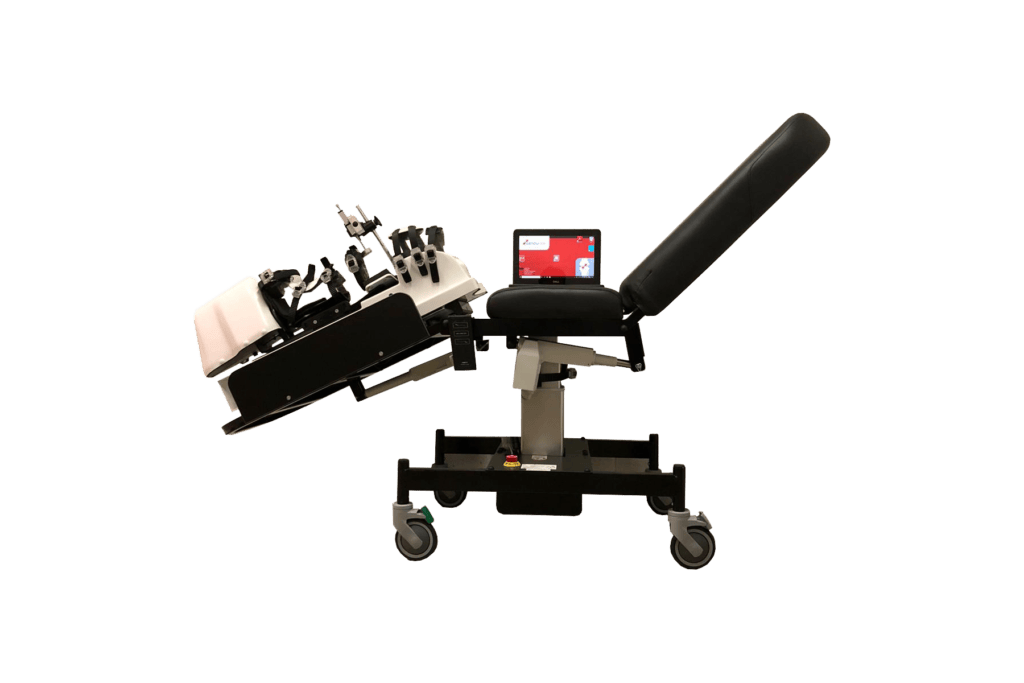
Dyneelax Arthrometer Characteristics
- Medical Device: High-Tech Robotic Knee Laxity Arthrometer
- Knee Dynamic Assessments Performed:
- Application of Anterior Tibial Translations to measure the state of resistance of the ACL.
- Inducement of Lateral Tibial Rotations to measure the state of resistance of knee ligamentous structures.
- Inducement of Medial Tibial Rotations to measure the state of resistance of knee ligamentous structures.
- Software: LDA Software – A user-friendly software developed by Genourob for optimal objective testing of the knee ligaments with an easy to access exportable database.
- Examination Couch: LDA Couch – An examination couch designed to mount the Dyneelax for optimal testing of the knee ligaments. Different knee flexions can be selected for different tests.
- Population: Wide range of normative data, from pediatric through adults, ages 8 – 100.
- After Sale: Excellent after-sale support, installation and continuing education programs.
- Warranty: 2 year-warranty.
- Shipment: Worldwide.
Dyneelax Knee Arthrometer
1) ACL Diagnosis
2) ACL Prevention
3) ACL Rehabilitation
4) ACL Follow-up
Why Knee Arthrometers Over MRI?
Sensitivity: A Recent study (DOI: 10.1016/j.knee.2023.03.017) has shown GNRB & Dyneelax knee arthrometers exhibit superior sensitivity in diagnosing partial ACL ruptures. Where MRIs might miss or misinterpret the minutiae of a partial rupture, these knee arthrometers catch them.
Cost-Efficient Diagnosis (90% Cost Reduction)
Economic benefits with reduced costs for both practitioners and patients.
Instantaneous & More Accurate Diagnostics
Immediate & accurate results about the state of resistance of the ACL without waiting for imaging processing.
Non-Invasive Evaluation
Reduces the need for invasive diagnostic procedures.
Free Space in MRI Rooms
Creates room for individuals battling more severe pathologies, like certain forms of Cancers.
How is Dyneelax Knee Arthrometer used by Medical Practitioners?
Dyneelax: A 4 in 1 high-tech Medical Device
Diagnosis of ACL, AL-PM & AM-PL Corner Injuries
Harnessing Dyneelax, we redefine ACL injury diagnosis. Especially with partial ACL ruptures, surpassing MRI's capabilities. Enter a new era of confident, precise assessments. Your knee's health, unmistakably reaffirmed.
Rehabilitation of ACL, AL-PM & AM-PL Corner Injuries
Embrace Dyneelax's revolutionary technology as we guide you through the journey of ACL injury rehabilitation. Witness a transformation in recovery, where hope meets innovation. Rebuild with confidence and conviction.
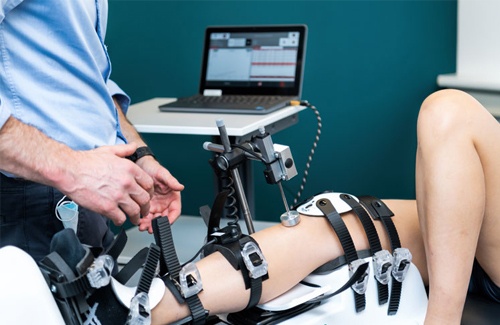
Prevention of ACL, AL-PM & AM-PL Corners Injuries
Utilizing the cutting-edge capabilities of Dyneelax, we unveil proactive strategies to prevent ACL injuries. Venture into a future where knee safety is prioritized. Protect and prevent, with assurance.
Follow-Up of the ACL, AL-PM & AM-PL Corners
With Dyneelax as our compass, embark on a path of vigilant follow-up care for ACL injuries. Enter a realm where progress is measured, and healing is tracked with precision. Your journey, our commitment.
DOI: 10.1016/j.knee.2023.03.017

DOI: 10.1016/j.medntd.2023.100254
In which Healthcare Departments is Dyneelax utilized?
What defines Dyneelax as a crucial asset in top-tier facilities and what features does it offer?
Enhanced Objective Knee Ligament Evaluation through Dynamic Assessments
Utilize the exactness of our arthrometer’s objective data to gain a comprehensive understanding of the resistance conditions specific to the ACL, the AM-PL & the AL-PM Corners.
The Dyneelax knee arthrometer pioneers a new era in knee laxity examination by delivering quantifiable insights, markedly diminishing the subjectivity typically inherent in such appraisals. Its distinctive feature lies in its capacity to measure both tibial translation and rotation, granting a comprehensive view of knee laxity—2023 – Theo Cojean – A study on sensitivity, repeatability, and reproducibility utilizing a leg prototype of the newly introduced knee arthrometer, Dyneelax.
These objective assessments are vividly presented through graphical displays that depict the compliance trajectories when varying forces are exerted on the tibia. This capability is instrumental in distinguishing between normative and pathologic knees. Additionally, the precision with which Dyneelax discerns knee stability via automated anterior drawer tests, coupled with its prowess in dissecting rotational instability, differentiates it from market counterparts such as the KT-1000 and KT-2000.
In 2023, a study by Theo Cojean highlighted Dyneelax's sensitivity, repeatability, and reproducibility. Furthermore, studies from 2012 Collette et al., 2017 Jenny et al., 2022 Kayla Smith, and 2023 Magdic have validated the efficiency of GNRB in diagnosing ACL ruptures, reinforcing their clinical significance. It should be noted that GNRB technology is incorporated in the Dyneelax.





A particular merit of the Dyneelax knee arthrometer is its diagnostic acumen, especially pertaining to partial ACL (anterior cruciate ligament) tears. Demonstrating a superior detection rate for partial ACL disruptions, it frequently outperforms the diagnostic sensitivity of MRIs—2023 – Theo Cojean – A prospective diagnostic study juxtaposing GNRB laximeter and MRI in clinical scenarios for detecting complete and partial ACL tears, validated via arthroscopy on 214 patients.
This heightened detection capability not only underscores Dyneelax’s diagnostic efficacy but also underscores its cost-efficiency and user accessibility over MRI, addressing certain MRI-associated diagnostic challenges for partial ACL tears.
More than just a precise and cost-saving device, Dyneelax promises user-friendliness, yielding results interpretable without intensive training. As a multifaceted instrument, it streamlines the diagnosis, prevention, rehabilitation, and follow-up of patients with knee ligament concerns. With its objective, sharp, and straightforward results, Dyneelax emerges as a prime alternative for diagnosing partial ACL tears, presenting an efficient, economical, and user-centric substitute for MRI.
Precision & Reproducibility: The Dyneelax Arthrometer's Credibility in Consistent Knee Laxity Assessments
The consistency inherent in the results produced by the Dyneelax knee arthrometer attests to its steadfast reliability across multiple evaluations. This pronounced reproducibility (Théo Cojean Study 2023) is cultivated through the incorporation of pinpoint sensors and well-delineated guidelines, fostering user simplicity and markedly elevating test precision. A standout attribute of Dyneelax is its adeptness at replicating the same starting conditions for each test, ascertaining the patient’s accurate positioning every time an assessment is undertaken. Such scrupulous command over the testing environment facilitates a direct comparison of outcomes over durations, becoming crucial in long-term research and post-operative assessments following Anterior Cruciate Ligament (ACL) repair.
Such uniformity in testing conditions and the consequent data consistency not only vouch for Dyneelax’s trustworthiness but also enhance its reputation within the medical fraternity. A testament to this is seen in studies like the one by Klasan, where Dyneelax was juxtaposed with the KT1000 knee arthrometer and was found to have a precision reminiscent of advanced navigation systems. A 2023 research initiative by Renata Vauhnik in Slovenia highlighted the Dyneelax knee arthrometer’s top-tier reproducibility, endorsing its significance in offering dependable, repeatable, and unbiased knee laxity assessments. Receiving consistent endorsements from both clinical and scholarly spheres, Dyneelax stands as an indispensable asset for healthcare practitioners seeking meticulous and consistent evaluations of knee laxity.
Safe ACL Assessments with Dyneelax
The safety protocols embedded in the Dyneelax knee arthrometer, particularly following Anterior Cruciate Ligament (ACL) reconstruction surgery, are pivotal to guarantee the ongoing health and systematic recuperation of patients. A prevalent apprehension among healthcare experts pertains to the potential jeopardy of harming the ACL graft during assessment. Nonetheless, it’s imperative to recognize that the forces enacted by Dyneelax stay well within permissible and endurable thresholds. For perspective, research indicates that routine motions such as walking can exert loads close to 350N on the ACL (Download Nagura Study – 2006 or DOI: 10.1123/jab.22.4.305). Contrarily, Dyneelax judiciously applies a mere 100N of force to the tibia when evaluating the post-operative ACL graft, thus substantially negating graft harm possibilities.
For pre-surgical evaluations, the applied force might peak at 200N to inspect knee vulnerabilities, still preserving a considerable safety buffer. With Dyneelax’s forces accurately modulated via computational oversight, it assures a regimented and secure testing milieu. Such precision not only vouches for patient safety but also facilitates meaningful early post-surgical graft examinations, meticulously tracking the recuperative trajectory. Bertrand Semay’s 2016 study (DOI: 10.1016/j.rehab.2016.07.045) focuses on the evolution of anteroposterior laxity in ACL reconstruction patients, measured by the GnRB device at 6, 9, and 12 months post-surgery. It underscores the importance of monitoring ligament laxity for effective rehabilitation.
Furthermore, Nouveau’s 2017 research (DOI: 10.24966/ORP-2052/100035) investigates the impact of early rehabilitation activities on the stiffness of ACL grafts, emphasizing the significance of balancing early mobilization with graft healing. Another interesting study is from Forelli in 2023 (DOI: 10.26603/001c.73031). It introduces a comprehensive rehabilitation approach, particularly for soccer players, stressing the need for a blend of clinical and sport-specific assessments to ensure a successful and safe return to play. These studies collectively advance the understanding of ACL injury management from surgical recovery to customized rehabilitation.
These scientific papers clearly state that the insights derived from these tests are pivotal, affording clinicians valuable feedback during the crucial recuperative window post-surgery. Such data-driven knowledge aids in crafting tailored rehabilitation blueprints, anchoring optimal knee stability for the foreseeable future. Additionally, Dyneelax’s motor-driven attributes sanction evaluations as soon as one month post-procedure, administering minimal forces and torques, thus upholding a thorough and risk-free assessment paradigm. The intricate architecture and disciplined functionality of Dyneelax epitomize its dedication to patient welfare, solidifying its standing as an integral and reliable asset in contemporary knee ligament diagnostics and care.
Integrated Software and Assured Durability: Our Organizational and Warranty Commitments

Our devices’ impeccable synergy with the proprietary LDA software highlights our unwavering commitment to seamless operational excellence. Crafted meticulously by Genourob, the LDA software is quintessential for executing tests with the knee arthrometers, embodying the perfect amalgamation of functionality and user-centric design. Beyond simplifying the testing paradigms, this software serves as a comprehensive repository, methodically archiving every patient evaluation performed. As outcomes are digitized and auto-archived, the intricacies of data management are significantly alleviated. This streamlining not only bolsters administrative proficiency but also fortifies communication avenues, ensuring pivotal data is always at hand and securely retained.
Our steadfast confidence in the resilience and reliability of our devices is amplified by the provision of a 2-year warranty. This warranty is a reflection of the fastidious craftsmanship and exacting quality checks that permeate our production process. Clients can rest easy, aware that their investment is in a device adeptly crafted to meet the challenges of relentless clinical usage, and further cushioned by the assurance of warranty coverage. Such warranty provisions exemplify our relentless drive towards client satisfaction and our immutable commitment to delivering peerless quality products.
What are the Technical Specifications of Dyneelax?
Main Specifications
- The medical device is a device to help diagnose partial or total lesions of the knee’s cruciate ligaments and peripheral collagenous structures
- Must be used by a health professional.
- The device can be used to help with diagnosis, to follow the evolution of a pathology or a ligamentoplasty (cruciate ligament operation).
- The principle of the device is double as it allows:
- To either to apply a thrust force on the calf thanks to an articulated mechanical system and to record the displacement of the tibia in relation to the femur for each force thanks to a sensor placed on the Anterior Tibial Tuberosity (ATT).
- Or to induce a force of internal or external rotation of the tibia in relation to the femur and to measure the resistance of the peripheral collagenous structures of the knee to this imposed rotation.
- Measurements are taken on both lower limbs and the analysis of the lesions can be done by observing the difference between these two values and especially by comparing the slope of the curves obtained.
- The system includes a 3-level safety system based on mechanic and software controlled mechanism.
Software Specifications
- The system includes a 3-level safety system based on mechanic and software controlled mechanism
- The software ensures optimal use of the product to avoid handling errors. Very intuitive and easy to use, the operator is guided on the different parameters to be validated before launching the tests.
- The system allows for easy register of patient
- The software should automatically apply chosen test
- The software help to practice a test with the same parameter from the most recent examination
- The software offers easy-to-read graphs with colours accompanied by a tables chart
Standard Accessories
- Operating instructions
- Computer/tablet
- Software
- Power cable
- USB cable
Optional Accessory
- PCL Option
Technical Data
- Power Supply: Input Voltage 230 VAC, Input Frequencies 50 or 60 Hz
- Dimension (standard position): 0,60 x 2 x 1,5 m (600mm x 2000mm x 1500mm)
- Weight: 100Kg
- Maximum Patient Weight: 150Kg
Dimension Details
For the height, in “classic” test configuration, there is dimensions of 205 * 65* 150 cm (tolerance ±5 according to the user setting).
The differences on the height are explained according to the adjustment of the table (with the jack in low position, we are at 95 cm and in high position we are at 170 cm). Afterwards, if we consider the height up to the seat where the patient sits, we have in min 65 cm and in max 105 cm.
The differences on the width are explained by the measurement taken on the ground and not on the dimension of the patient support. The support is 50 cm ±5 but on the ground the device is 60 cm ±5.
For the angles, the backrest is from 25° to 85°, but for obtaining a good reason with a patient we suggest it should be from 60° to 85°, and leg support is 0 to 40° without the device but when we add the device, it adds 15° so it is from 0 to 55° (all angles have a tolerance of ±2)
For the transport (delivery), it is carried out by means of a packaging of size 80*160*130 cm (tolerance related to the wood ±4)
