Dyneelax - Knee Arthrometer
Knee Ligament Structures Analysed: ACL, AM-PL & AL-PM Corners
Tibials Movements: Anterior /posterior Translation & Medial / Lateral Rotation
The Dyneelax knee arthrometer is an essential medical device for detailed joint stability and ligament evaluations, ideal for various medical specialists. With its ergonomic build and sophisticated measuring system, it offers dependable results and an intuitive evaluation process, raising the bar for precision in knee diagnostics & rehabilitation.
This device is an innovative medical instrument tailored for the detection, prevention, rehabilitation, and continuous observation of patients suffering from knee ligament impairments. It delivers meticulous evaluations of knee laxity by executing automated anterior drawer evaluations and rotational instability examinations, with a primary emphasis on the anterior cruciate ligament (ACL).
What is Dyneelax?
Unlock unparalleled clarity in knee ligament assessment with Dyneelax: where precision meets simplicity, redefining the gold standard in ligament diagnostics & Treatment.

Injury Diagnosis (ACL tears, including partial tears)

Surgical planning / medial-side & rotational control

Post-op monitoring / graft & laxity evolution

Imaging Correlation (MRI biomarkers + objective laxity)
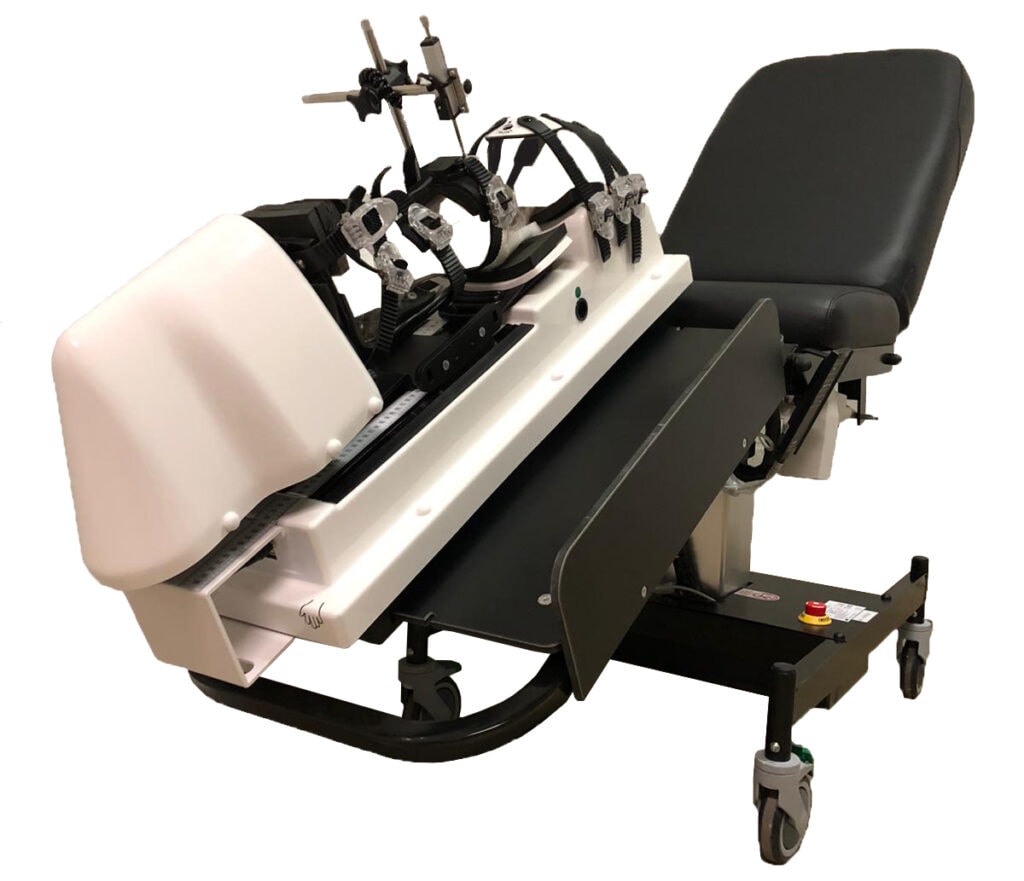
Anterior/Posterior Tibial Translation
Diagnose Partial & Complete ruptures of the Anterior Cruciate Ligament (ACL) and the Posterior Curciate Ligament (PCL).
Measure the state of resistance of the ACL/PCL by applying automated anterior/posterior tibial translation.
Results are shown under the form of compliance curves (force/displacement curves) + Tables Chart with displacement data.
Stop measuring laxity alone
Measure Ligament Comliance
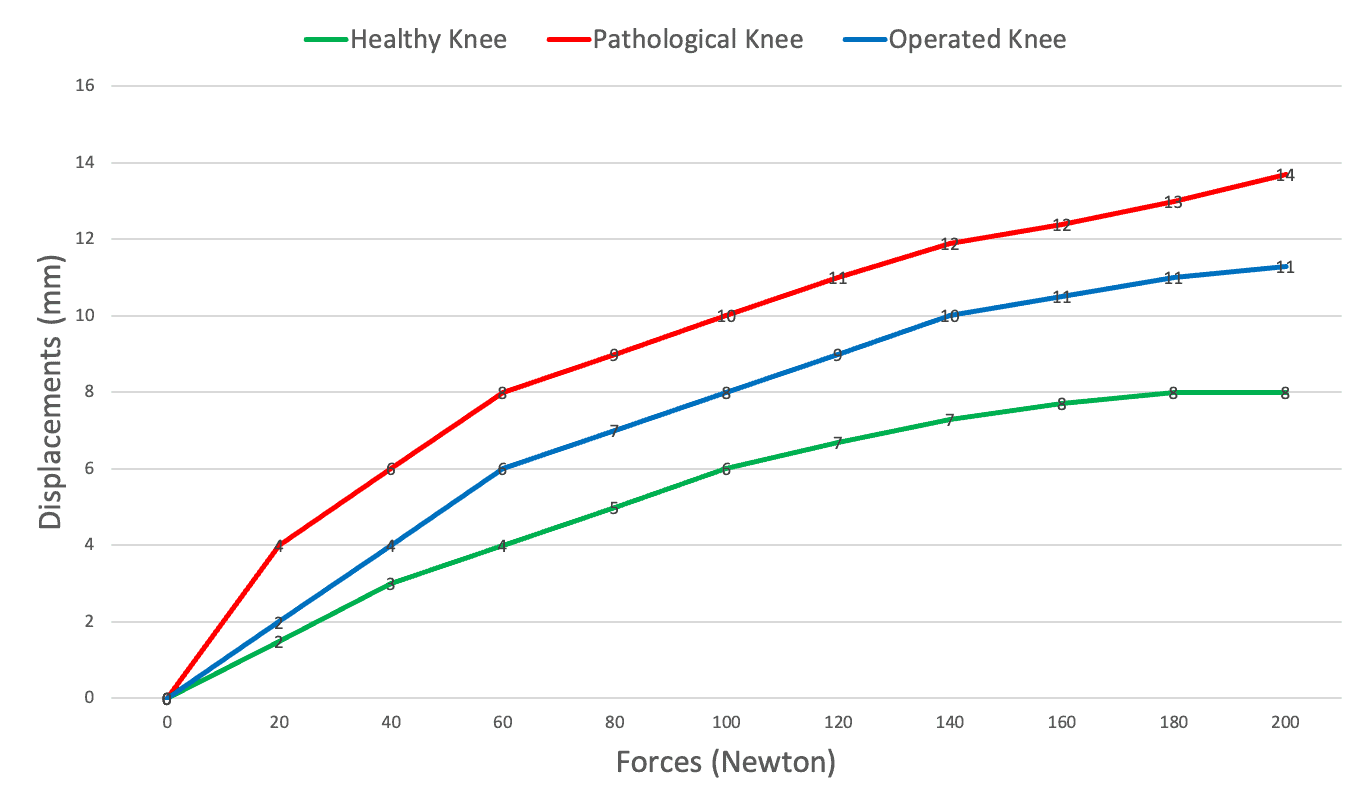
Why does compliance outperform laxity alone?
Laxity is a single value. Compliance captures the full force–displacement curve, revealing how the ligament behaves under load—for clearer diagnosis, planning, and follow-up.
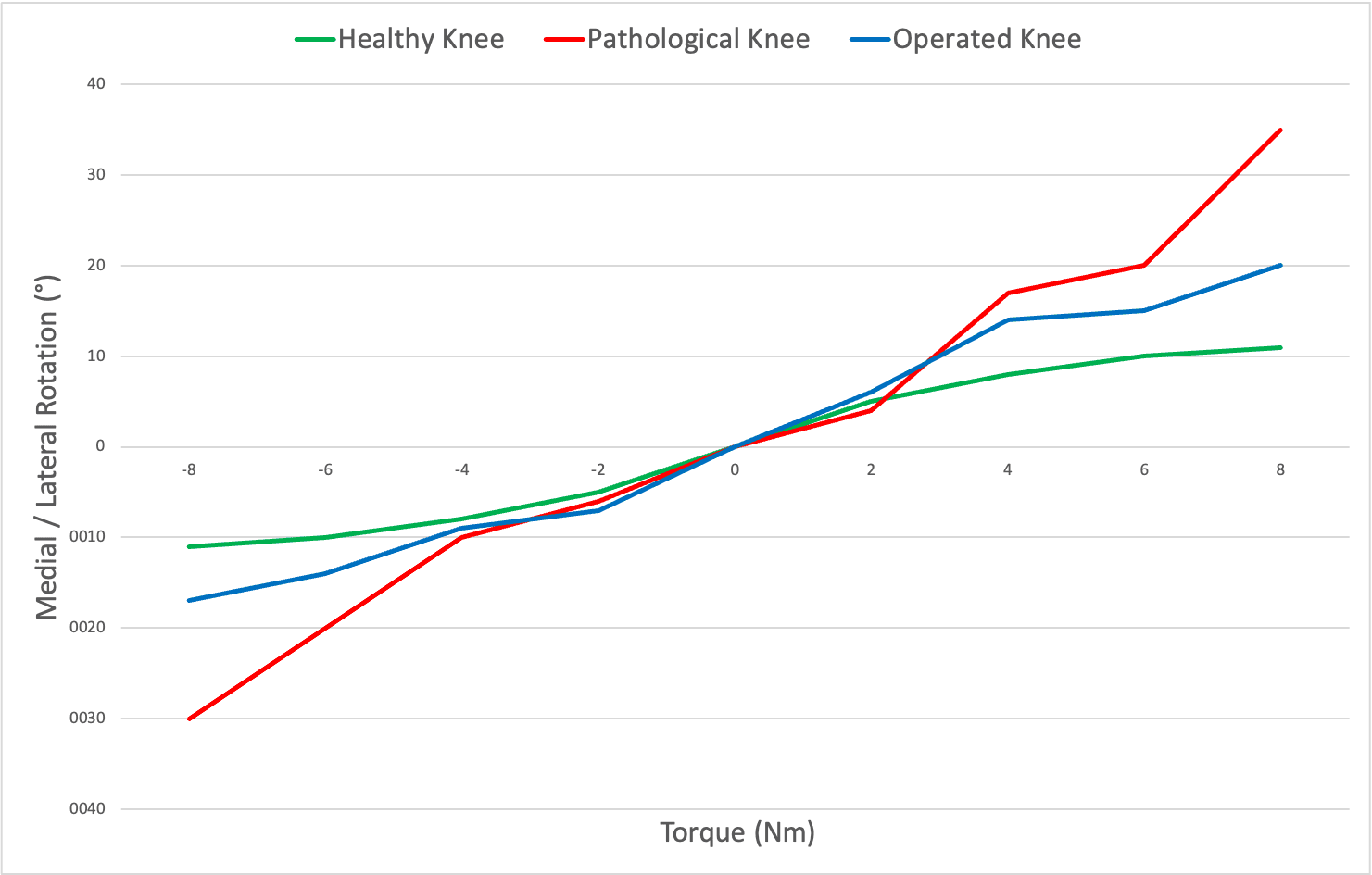
Measure Medial/Lateral Tibial Rotation
Evaluate structures surrounding the ACL/PCL by applying robotic tibial medial / lateral rotations.
Correlate these dynamic measurements with static MRI findings to precisely tailor the surgical strategy.
Results are also shown under the form of curves (degree/torque curves) + Tables Chart with rotation data.
Clinical Benefits Across the Entire Knee Ligament Pathway
Identify partial ACL tears with dynamic testing and objective measurements.
Turn instability into measurable data—not just static imaging.
Combine Dyneelax dynamic stability data with MRI to better identify which structures are compromised.
This supports more precise surgical decisions and a patient-specific reconstruction strategy.
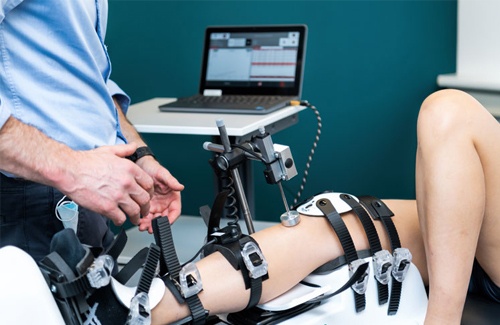

Thanks to our system's high reproducibility, we maximize the effectiveness of early detection and preventive strategies, paving the way for ongoing follow-ups.
.
Enable graft monitoring after ACL reconstruction through serial, objective stability measurements.
Optimize key milestones: isokinetic strength testing, rehab progression, and safer return-to-sport timing.

DOI: 10.1002/jeo2.70026
DOI: 10.1016/j.medntd.2023.100254





What are the Technical Specifications of Dyneelax?
Main Specifications
- The medical device is a device to help diagnose partial or total lesions of the knee’s cruciate ligaments and peripheral collagenous structures
- Must be used by a health professional.
- The device can be used to help with diagnosis, to follow the evolution of a pathology or a ligamentoplasty (cruciate ligament operation).
- The principle of the device is double as it allows:
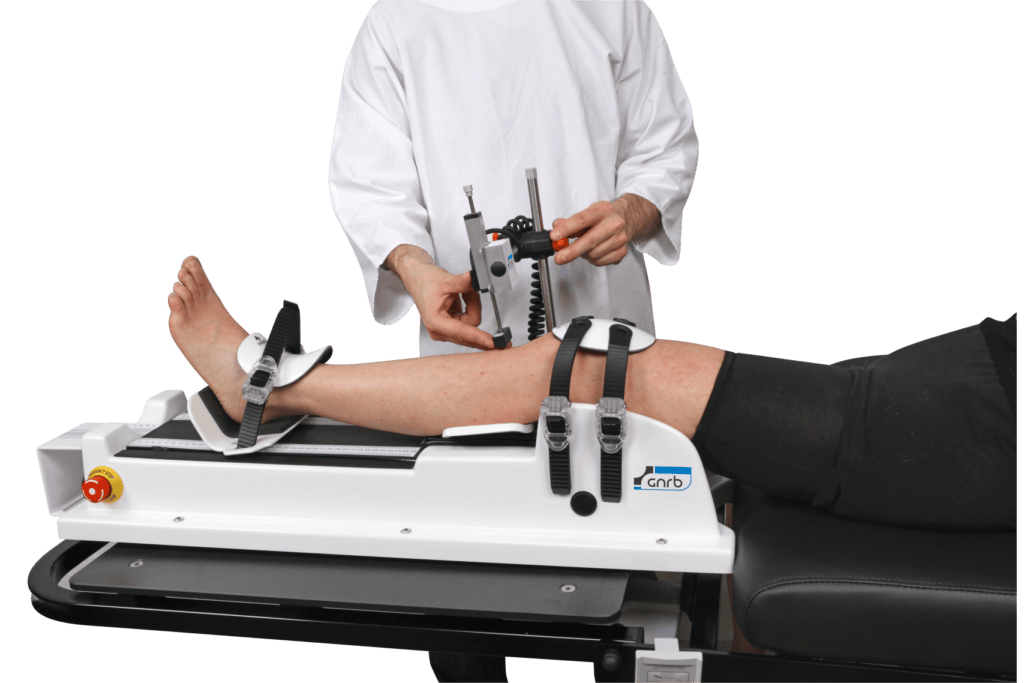
- To either to apply a thrust force on the calf thanks to an articulated mechanical system and to record the displacement of the tibia in relation to the femur for each force thanks to a sensor placed on the Anterior Tibial Tuberosity (ATT).
- Or to induce a force of internal or external rotation of the tibia in relation to the femur and to measure the resistance of the peripheral collagenous structures of the knee to this imposed rotation.
- Measurements are taken on both lower limbs and the analysis of the lesions can be done by observing the difference between these two values and especially by comparing the slope of the curves obtained.
- The system includes a 3-level safety system based on mechanic and software controlled mechanism.
Software Specifications
- The system includes a 3-level safety system based on mechanic and software controlled mechanism
- The software ensures optimal use of the product to avoid handling errors. Very intuitive and easy to use, the operator is guided on the different parameters to be validated before launching the tests.
- The system allows for easy register of patient
- The software should automatically apply chosen test
- The software help to practice a test with the same parameter from the most recent examination
- The software offers easy-to-read graphs with colours accompanied by a tables chart
Standard Accessories
- Operating instructions
- Computer/tablet
- Software
- Power cable
- USB cable
Optional Accessory
- PCL Option
Technical Data
- Power Supply: Input Voltage 230 VAC, Input Frequencies 50 or 60 Hz
- Dimension (standard position): 0,60 x 2 x 1,5 m (600mm x 2000mm x 1500mm)
- Weight: 100Kg
- Maximum Patient Weight: 150Kg
Dimension Details
For the height, in “classic” test configuration, there is dimensions of 205 * 65* 150 cm (tolerance ±5 according to the user setting).
The differences on the height are explained according to the adjustment of the table (with the jack in low position, we are at 95 cm and in high position we are at 170 cm). Afterwards, if we consider the height up to the seat where the patient sits, we have in min 65 cm and in max 105 cm.
The differences on the width are explained by the measurement taken on the ground and not on the dimension of the patient support. The support is 50 cm ±5 but on the ground the device is 60 cm ±5.
For the angles, the backrest is from 25° to 85°, but for obtaining a good reason with a patient we suggest it should be from 60° to 85°, and leg support is 0 to 40° without the device but when we add the device, it adds 15° so it is from 0 to 55° (all angles have a tolerance of ±2)
For the transport (delivery), it is carried out by means of a packaging of size 80*160*130 cm (tolerance related to the wood ±4)

